COMPUTATIONAL FLUID DYNAMICS OF FETAL RIGHT VENTRICLE BASED ON PATIENT-SPECIFIC ULTRASOUND IMAGES
For the full details of the study, please refer to published paper here.
INTRODUCTION
Congenital cardiovascular diseases affects between 0.6-1.9% of births [1], sometimes with devastating consequences. It includes conditions such as Hypoplastic Leart Heart Syndrome, Tetralogy of Fallot, and Persistent Truncus Arteriosus, all of which requires urgent surgery at birth. Recent animal studies have shown that altered fluid mechanical blood flow environment of the embryonic / fetal circulation may be the cause of congenital cardiovascular diseases [2-4], prompting studies on fetal circulatory fluid mechanics.
Imaging or simulating fluid flow in the human fetal heart is difficult as 4D imaging modality to obtain fetal heart dynamics reliably is challenging. For example, CT cannot be used on fetuses due to radiation, while MRI requires synchronization with fetal heart rate, which is challenging.
We recently developed a new technique, using the Spatio-Temporal Image Correlation (STIC) 4D ultrasound imaging as the imaging modality [5], to circumvent this problem Our technique involved performing patient-specific Computational Fluid Dynamics (CFD) simulations based on STIC images, so as to elucidate the fluid dynamics of human fetal hearts.
In the current study, we applied this technique to two human fetal hearts at 20-week gestation to elucidate the fluid mechanics within the right ventricle. We also performed energy balance analysis to understand whether the diastolic vortex rings play a role in conserving kinetic energy of flow from one cycle to the next.
METHODS
The 4D ultrasound images of two 20 weeks old human fetal hearts were obtained using the Voluson 730 ultrasound machine (GE Healthcare Inc.) under STIC (Spatio-Temporal Image Correlation) mode. Images were exported as a 3D stack of images over 29 time points.
The images were first re-sampled along multiple slices from the tricuspid inlet to the pulmonary outlet. The luminal cross-sectional area of the right ventricle was segmented using custom written lazy snapping algorithm over all time points, over all slices.
The right ventricle chambers were segmented in 3D using the open-source program, VMTK, for 3D segmentation, for CFD analysis. A mathematical model was obtained through a 2D Fourier Transformation of the radius amplitudes measured from the 3D segmentation. It was then utilized to control dynamic mesh wall motion in the CFD simulations.
CFD simulation is done with FLUENT 16.0 (ANSYS Inc.) in the central HPC cluster. The boundary conditions were such that during diastole, the outlet was a zero velocity wall and the inlet was a zero pressure inlet. During systole, the outlet was a zero pressure outlet and the inlet was a zero velocity wall. Non-Newtonian fluid following the Carreau model was utilized, and the simulations were performed for four complete cardiac cycles to remove initial condition artefacts. The CFD simulation consists of 1 million cells and it takes about 1 day to complete the simulation for four complete cardiac cycles using eight cores.
RESULTS AND FINDINGS
Figure 1 shows the results of wall motion modeling for use in CFD. Results showed a good match with the 3D reconstruction from the ultrasound images. Figure 2 shows the results of the simulations, in terms of the spatial pressure variations and wall shear stresses on the walls of the fetal right ventricle, and the vortex dynamics within the ventricle. From figure 2, it was also observed that the location of high wall shear stress coincides with the location of high vorticity, as the vortices generated velocity tangential to the heart wall, which led to elevated wall shear stresses.
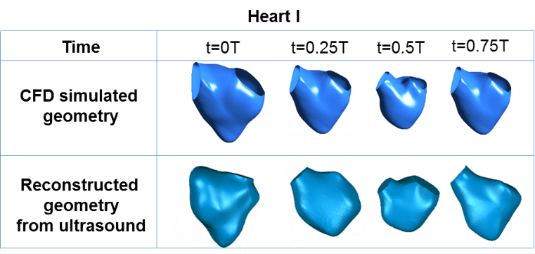
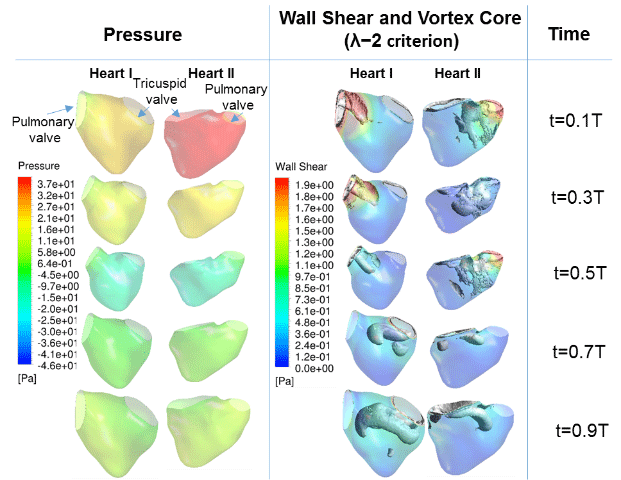
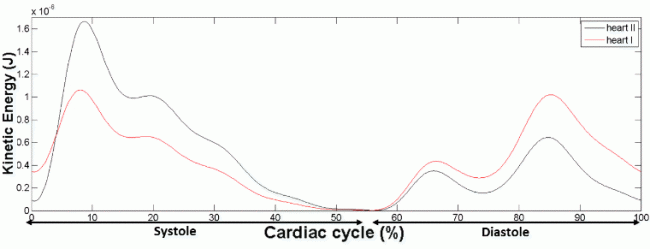
Figure 3 shows our quantification of kinetic energy (KE) of right ventricular flow. Elevation of KE was observed during systolic outflow and during both the E- and A-wave of diastolic inflow. The results showed that an average of 24% of the peak kinetic energy from diastole is transferred to the subsequent systole.
For the first time, we performed patient-specific CFD simulations of the human fetal right ventricle based on 4D clinical ultrasound imaging. Our simulations revealed interesting fluid dynamics in the human fetal right ventricle, where two independent vortex rings were observed to merge over the course of diastole. We hypothesize that the characteristics of these vortices can be used as markers for heart diseases, like that proposed for adult hearts.
Results obtained in our studies showed that the ventricular vortices were driving forces behind the elevation of shear stresses during diastole. It is thus important to study their features further.
ACKNOWLEDGEMENTS
We thank the Singapore Ministry of Health, National Medical Research Council Grant Number NMRC/BNIG/2020/2014 (PI: Yap) for their funding. The full paper was submitted for the Summer Biomechanics, Bioengineering and Biotransport Conference (B3C2016), June 29 –July 2, National Harbor, MD, USA
References:
- Hoffman, J.I. and S. Kaplan, The incidence of congenital heart disease. J Am Coll Cardiol, 2002. 39(12): p. 1890-900.
- Hove, J.R., et al., Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature, 2003. 421(6919): p. 172-7.
- Hogers, B., et al., Intracardiac blood flow patterns related to the yolk sac circulation of the chick embryo. Circ Res, 1995. 76(5): p. 871-7.
- Tobita, K. and B.B. Keller, Right and left ventricular wall deformation patterns in normal and left heart hypoplasia chick embryos. Am J Physiol Heart Circ Physiol, 2000. 279(3): p. H959-69.
- Lai, C.Q., et al., Fluid mechanics of blood flow in human fetal left ventricles based on patient-specific 4D ultrasound scans. Biomechanics and modeling in mechanobiology, 2015: p. 1-14.

