INTERFACIAL INTERACTION BETWEEN METALS AND MONOLAYER MoS2 REVEALED BY FIRST-PRINCIPLES CALCULATIONS
The electrical contact between metals and semiconductors plays an increasingly important role in determining the electronic device performance when the device size is approaching nanoscale. Transition metal dichalcogenide monolayers (TMD) is a promising alternative to Si as channel material. However, it is challenging to obtain a low contact resistance for electrical contacts between metals and two-dimensional (2D) TMD materials. Even for low work function metals, with a work function comparable to the electron affinity of TMD monolayers, a sizable contact barrier height still occurs, which is due to the Fermi level pinning effect induced by the interaction between the metal and the TMD monolayer.
In this study, density-functional theory (DFT) based first-principles calculations were carried out to study the interfacial interaction between metals and monolayer MoS2, in which the Vienna ab initio simulation package (VASP5.4.1) was used with the projector augmented wave (PAW) pseudopotentials, the Perdew-Burke-Ernzerhof (PBE) exchange-correlation functionals, and plane-wave basis.
Our calculations show that for high work-function metal (Ni) on the monolayer MoS2, the interfacial interaction is strong, as supported by large adsorption energy, the formation of interfacial bonds, and significant charge redistribution on the MoS2 basal plane in Fig. 1.
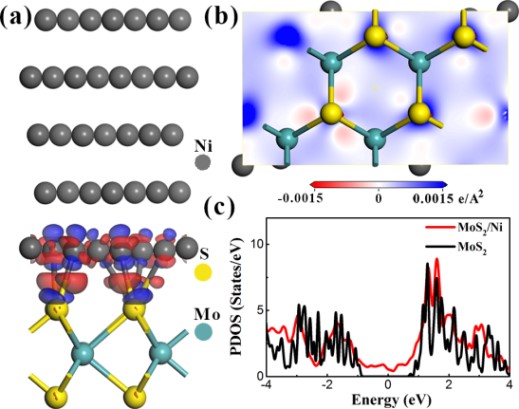
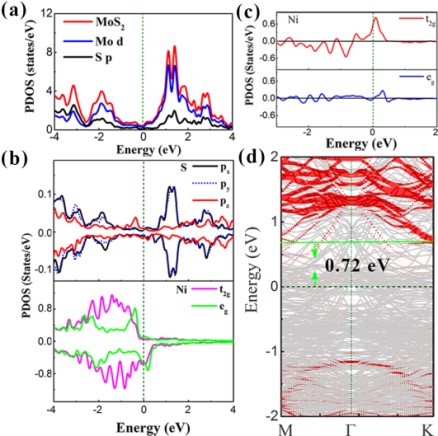
When in contact with chemically more reactive Ni, the Mo-S bonds are weakened because of the formation of interfacial Ni-S bonds. The low lying unoccupied electronic states derived from Mo d orbitals spread into the band gap, forming the metallic states. These mid-gap states pin the Fermi level partially, resulting in a large Schottky contact barriers (0.72 eV) between Ni and monolayer MoS2.
Our calculations further show that when an additional MoS2 layer is introduced as a buffer layer as shown in Fig. 3(a), the strong interaction between Ni and the MoS2 channel layer can be decoupled. Thus, the strong interaction is confined at the interface between Ni and the MoS2 buffer layer, and does not extend into the MoS2 channel layer, as supported by the averaged charge density in Fig. 3(b). The MoS2 channel layer retains its semiconducting character, as well as a much reduced Schottky contact barriers (see Fig. 3(d)).
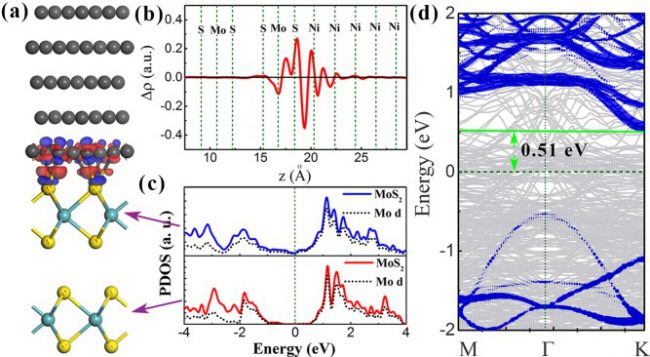
We wish to point out that the SBH can be further reduced to 0.29 eV by using the low work function metal Ti as the contact metal. Our results demonstrate the potential of using a MoS2 layer as an electrode buffer layer, and develop a simplified approach to improve contact performance between metals and 2D semiconductors. Due to the similar surface chemistry of 2D materials, our results might shed light on the understanding and integration of metal contacts with other 2D semiconductor materials.
Reference
Chai J. W., et al. Tuning Contact Barrier Height between Metals and MoS2 Monolayer through Interface Engineering, Adv. Mater. Interfaces 2017, 1700035.

